Background.
Sickle cell disease (SCD) is a group of blood disorders that results from point mutations causing different hemoglobinopathies, including hemoglobin SS disease, hemoglobin SC disease, and sickle cell beta-thalassemia. According to the Centers for Disease Control and Prevention (CDC), it is estimated that SCD affects 100,000 Americans. SCD occurs among one out of every 365 African-America births, and one out of every 16,300 Hispanic births. Approximately, one in 13 African-America babies is born with sickle cell trait. Despite being a common disease, patients with SCD have less access to comprehensive team care than patients with other genetic diseases. The state of Nebraska has a population close to two million, where the three most common races are Caucasians (78.1%), Hispanic (11.4%) and African American (5.2%). Despite the racial distribution, SCD is not uncommon in Nebraska. Herein, we report the first database of SCD in the state of Nebraska by reviewing the records of the two biggest tertiary care centers in the state: The University of Nebraska Medical Center (UNMC) and Children's Hospital & Medical Center.
Methods:
This is a retrospective study. After an IRB approval, we retrospectively reviewed charts of 358 patients who had an ICD-10 code related to SCD and had their care at UNMC or Children's Hospital & Medical Center since January 2014. Inclusion criteria included any patient, regardless of age, who had a confirmed diagnosed of SCD regardless of the genotype. Data was collected to create a comprehensive data base for both adults and children. Children were defined as any individual who is following with pediatric hematology as an outpatient or was born on or after January 1, 2002.
Results:
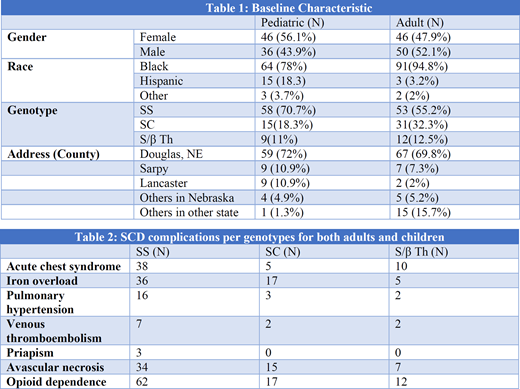
A total of 358 patients were reviewed with 355 patients included in the study. Ninety six of them were adults with SCD (mean age of 28.9 years) while 82 were children with SCD (mean age 5.7 years), and 178 patients with sickle cell trait. Table 1 summarizes the demographics for adults and children with SCD. Only 30 out 96 patients from the adult group were employed. Sixty three adult patients were prescribed hydroxyurea compared to 32 children. Regarding simple red blood cell transfusion, the adult group had a median of 2.1 units/year (0-99.2 units/year) which was less than the pediatric group 5.8 unit/year (0-95.6). Twenty one adult patients had alloantibodies, with Anti-E as the most common , while only three children had alloantibodies. Table 2 summarizes the SCD complications for both adults and children per genotype. Our study showed that having SS genotype was associated with higher risk for acute chest syndrome (p= 0.0023) and iron overload (p=0.039). Moreover, SS genotype was predictor of more emergency room (ER) visits and admissions compared to the SC genotype. Since January 2014, the mean number for clinic visits was higher for children compared to adults (30 vs 14.3 visits, p<.001). However, the mean number for ER visits was higher in adults compared to children (8.1 vs 3.8 visits, p<.001). Also, adults spent more days in the hospital with a median of 20 days (1-748 days) compared to pediatrics who had a median of four days (1-94 days). The probability of admission once presenting to the ER was also higher in the adults group compared to pediatrics group (0.56 vs 0.44). During the study period, six adults patients died and none from the pediatric group.
Conclusion:
SCD and its complications represents a serious issue in the state of Nebraska. It is also associated with high acute health care utilization. Our study showed that most of the cases are in the urban areas of the state of Nebraska. Also, we noticed that adults have more demands and lack of care compared to children, and they also don't follow in clinics as children do. This project represents the first step in the plan to improve the care of patients with sickle cell disease in Nebraska, through establishing a comprehensive data base and a comprehensive sickle cell disease clinic for both adults and children.
Gundabolu:BioMarin: Consultancy; Bristol Myers Squibb pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal